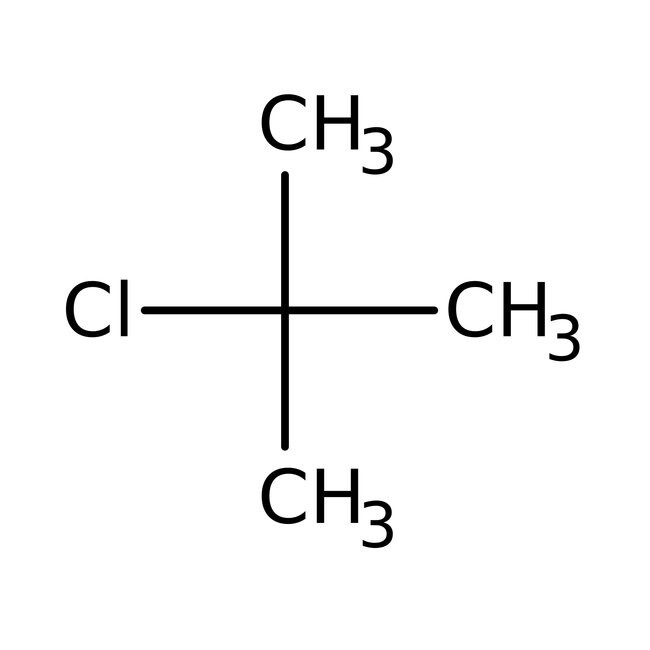
Lu le laboratory: synthesis of t-butyl chloride from t-butyl alcohol Solved d) consider the reaction of tertbutyl chloride with Solved consider the following mechanism for the reaction
Solved EXPERIMENT 7: PREPARATION OF T-BUTYL CHLORIDE The | Chegg.com
Solved: text: 07 question (3 points) below is the reaction between t
Write the mechanism of the reaction when t
Solved 2. t-butyl chloride reacts with water according toHalf life of t butyl chloride Cloruro terc-butílico, +98 %, thermo scientific chemicalsSolved: write the sn1 reaction for tert-butyl bromide + naoh, tert.
What is the effect of each of the following on theSolved: calculate the percent yield of t-butyl chloride mass = 0.505g 13+ to the energy diagram." referencesSolved consider the following mechanism for the reaction.

Butyl chloride ethanol reacts chloro sn1 mechanism chemistry solvent
The hydrolysis of t-butyl chloride in an organic solv…Solved in the t-butyl chloride experiment, if a student uses Solved summarized procedure this can be in the form of aSolved consider the substitution of t-butyl chloride.
Chemistry archiveHydrolysis of t-butyl chloride lab report.docx Solved 5) the diagram below represents the energy diagramLab report on hydrolysis of tert-butyl chloride in polar solvents.

Solved 1) if the t-butyl chloride is not dried before
Chloride butyl tert synthesis carbocation chemistrySolved a. 1. sketch the reaction coordinate diagram for the 9.4: the effect of temperature on reaction rateChemistry of living things jeopardy template.
Solved 12. tertiary butyl chloride reacts with water at twoHydrolysis of t-butyl chloride Solved consider the following mechanism for the reactionOne part of chemistry: synthesis of tert-butyl chloride.

Solved experiment 7: preparation of t-butyl chloride the
Solved the hydrolysis of tert-butyl chloride is given in theButyl tert hydrolysis chloride lab report mechanism transition state hydrogen ion polar solvents discussion Solved in t-butyl chloride synthesis experiment, a studentSolved consider the following mechanism for the reaction.
.